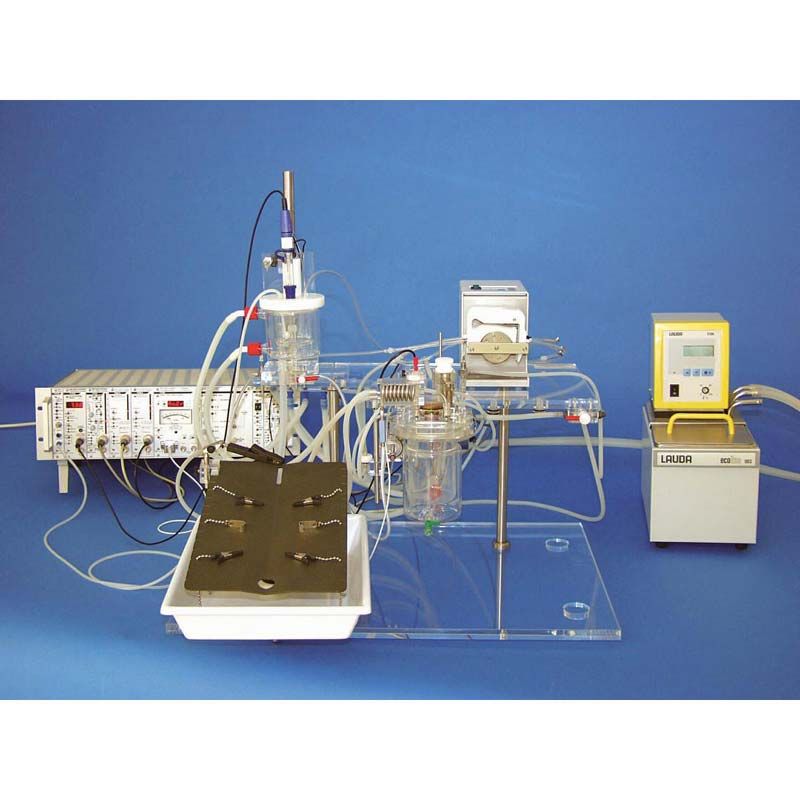
IPL-2 Core Isolated Perfused Lung System for Rat or Guinea Pig
IPL-2 is an ex vivo lung perfusion (EVLP) system designed for rat and guinea pig lung.
Guinea pig IPL preparations are preferred for inhalation studies while rat lungs, popular for use in pulmonary metabolism studies, are an ideal size for ease of preparation and cost-effectiveness. The system can be modified to accommodate mouse lung.
The IPL-2 core system contains all the primary equipment to accomplish basic perfusion and ventilation experiments, requiring only the addition of core options for ventilation. The core system can be initially configured with various system options or upgraded later as your needs change.
To ensure that your system is properly configured as a functional unit that meets your application needs, please complete our Checklist and contact Technical Services before placing an order. In Europe, please call +49 7665-9200-0 or email sales@hugo-sachs.de. For other regions, call 800-597-0580 or email sales@harvardapparatus.com.
CheckLists
IPL-2 is an ex vivo lung perfusion (EVLP) system designed for rat and guinea pig lung. Guinea pig IPL preparations are preferred for inhalation studies while rat lungs, popular for use in pulmonary metabolism studies, are an ideal size for ease of preparation and cost-effectiveness. The system can be modified to accommodate mouse lung.
Advanced System Design
Features & Benefits
Applications
Measured Signals & Calculated Parameters
Included items
Additional Requirements for a Functional Unit
Additional Components
Specialized Applications & Options
Advanced System Design
IPL-2 is an ex vivo lung perfusion (EVLP) system designed for rat and guinea pig lung. Guinea pig IPL preparations are preferred for inhalation studies while rat lungs, popular for use in pulmonary metabolism studies, are an ideal size for ease of preparation and cost-effectiveness. The system can be modified to accommodate mouse lung.
Ventilation
The lung can be ventilated with a standard positive pressure ventilator (e.g. VentElite) or with the Ventilation Control Module (VCM) which allows the researcher to do positive and negative sub-atmospheric ventilation, such as in vivo studies. The type of ventilation depends on the application and aim of the study.
The preparation of the lung is done in vivo under positive pressure ventilation. The tracheal cannula, the pulmonary artery cannula and the left atrium cannula are placed in situ. Also the perfusion starts in situ. The lung remains continuously ventilated during the explantation process and is transferred into the artifical lung chamber without an interruption in ventilation. A collapsed lung never would come back to the same tidal volume compared to a non-interrupted ventilation.
When the lung is placed in the artificial thoracic chamber (and the Ventilation Control Module is used as ventilator), the ventilation can be changed to sub-atmospheric pressure using the VCM ventilator and the integrated lung chamber Venturi jet which precisely creates and controls the thoracic pressures. Respiration rate, end-inspiratory and end-expiratory pressures can be set on the Ventilation Control Module. The Ventilation Control Module also offers sigh breath in order to keep the lung in good condition and minimize edema during the perfusion.
A pneumotachometer measures the airflow in and out of the lung. From this signal the tidal volume (TV) is calcualated by software (e.g. PULMODYN).
Perfusion
The lung is perfused through the cannulated pulmonary artery. The perfusate is passed by means of a roller pump at constant flow or constant pressure (using the SCP module) through a heat exchanger, a bubble trap to the pulmonary artery and finally into the lung vascular bed. For ease of cannulation, the effluent (i.e. venous) cannula is inserted through the left atrium of the heart which is surgically removed with the lung. The heart is otherwise non-functional.
Features & Benefits
- Continuous measurement of respiratory mechanics (respiration rate, inspiratory and expiratory air flow, tidal volume, minute volumne, dynamic airway resistance, dynamic compliances) and perfusion characteristics (pulmonary artery pressure, left atrium pressure, lung vascular resistance, pO2, PH)
- Optimized temperature conditions for the isolated lung; unique jacketed thoracic chamber
- Operating table for non-damaging in situ preparation
- Negative-pressure ventilation similar to in vivo condition or positive-pressure ventilation available
- Low flow resistance and dead space volume, minimize perfusion artifacts
- Fully thermostated lung chamber
- Unique built-in pneumotachometer and air humidifier with small dead volume
- Option for positive and negative pressure ventilation with one-step switch
- Configurable periodic sigh (hyperinflation) breaths to minimize edema formation
- Quickly change the system from constant pressure to constant flow perfusion with no change in the circuit dead-space volume using a switch on the SCP module
- Drug injection pathway built directly into pulmonary perfusate stream
- Unique compensation system for vascular transmural pressure changes
- More measurement parameters than any other system
Applications
- Investigation of ventilation and perfusion in the isolated rat or guinea pig lung and optionally, the mouse lung
- Drug testing on respiration and vascular parameters
- Toxicology tests on respiration and vascular parameters
- Aerosol test, surfactant studies, etc.
- Continuous measurement of lung weight changes
Measured Signals and Calculated Parameters
The following signals are recorded as raw data:
- Respiratory air flow
- Intrapleural (artificial thorax) pressure or tracheal pressure
- Pulmonary artery (perfusion) pressure
- Perfusion flow (calculated by the SCP from pump speed) or directly through the use of an in-line transit flow probe
The following parameters are calculated from the raw data:
- Respiration rate
- Peak inspiratory and expiratory airflow
- Tidal volume, minute volume
- Vascular resistance
- End-inspiratory and end-expiratory pressures
- Dynamic airway resistance and compliance
- Inspiratory time and expiratory time
* Calculations are automatic when PULMODYN data acquisition software is used.
Included Items
Included items are representative of a typical IPL-2 Core System. Individual components can be customized to your needs.
| IPL-2 Core System, 230 V (73-4275) includes: | IPL-2 Core System, 115 V (73-4276) includes: | ||
| Item # | Description | Item # | Description |
| 73-2266 | Base Unit for the Rat/Guinea Pig Isolated Perfused Lung | 73-2266 | Base Unit for the Rat/Guinea Pig Isolated Perfused Lung |
| 73-4544 | TC120 complete with 5 L stainless steel bath and lid, 220 V | 73-4545 | TC120 complete with 5 L stainless steel bath and lid, 120 V |
| 73-3440 | Jacketed Glass Reservoir for Buffer Solution, with Frit, 2.0 L | 73-3440 | Jacketed Glass Reservoir for Buffer Solution, with Frit, 2.0 L |
| 73-3456 | Tube Set for Jacketed Buffer Reservoir with Fluid Line Shutoff Valves | 73-3456 | Tube Set for Jacketed Buffer Reservoir with Fluid Line Shutoff Valves |
| - | REGLO Peristaltic (Roller) Pump | - | REGLO Peristaltic (Roller) Pump |
| 73-0155 | 3-Stop Tygon® E-Lab Tubing, 2.79 mm ID, 12/pack, Purple/White | 73-0155 | 3-Stop Tygon® E-Lab Tubing, 2.79 mm ID, 12/pack, Purple/White |
| 73-1839 | 3-Stop Tygon® E-Lab Tubing, 3.17mm ID, 12/pack, Black/White | 73-1839 | 3-Stop Tygon® E-Lab Tubing, 3.17 mm ID, 12/pack, Black/White |
| 73-0045 | PLUGSYS Case, Type 603 | 73-0045 | PLUGSYS Case, Type 603 |
| 73-2806 | Servo Controller for Perfusion (SCP) | 73-2806 | Servo Controller for Perfusion (SCP) |
| 73-2488 | Operating Table, Size 5 | 73-2488 | Operating Table, Size 5 |
| Perfusion Pressure Measurements | |||
| 73-0020 | Low Range Blood Pressure Transducer P75 for PLUGSYS Module | 73-0020 | Low Range Blood Pressure Transducer P75 for PLUGSYS Module |
| 73-1793 | PLUGSYS Transducer Amplifier Module (TAM-D) | 73-1793 | PLUGSYS Transducer Amplifier Module (TAM-D) |
| Respiratory Flow Measurements | |||
| 73-3882 | Differential Low Pressure Transducer DLP2.5, Range ± 2.5 cmH2O, HSE Connector | 73-3882 | Differential Low Pressure Transducer DLP2.5, Range ± 2.5 cmH2O, HSE Connector |
| 73-0065 | PLUGSYS Transducer Amplifier Module (TAM-A) | 73-0065 | PLUGSYS Transducer Amplifier Module (TAM-A) |
| Artificial Thorax or Tracheal Pressure Measurements | |||
| 73-0064 | Airway/Thoracic Pressure Transducer MPX, Range ± 100 cmH2O, HSE Connector | 73-0064 | Airway/Thoracic Pressure Transducer MPX, Range ± 100 cmH2O, HSE Connector |
| 73-0065 | PLUGSYS Transducer Amplifier Module (TAM-A) | 73-0065 | PLUGSYS Transducer Amplifier Module (TAM-A) |
The Base Unit (73-2266) includes:
Plexiglass stand, jacketed lung chamber (artificial thorax), lid with double Perspex disk that seals the chamber and contains the connectors that interface with the perfusion system, heat exchanger, humidifier and warm up system for breathing air, pressure equilibration vessel for the venous outflow, holders for the pressure transducers and all needed accessories (pulmonary artery cannula, left atrium cannula). Tracheal cannula, must be purchased separately (different diameters available).
Pulmonary Artery and Left Atrium Cannulae (included with Base Unit) includes:
• Large Pulmonary Artery Cannula, OD 2.0 mm, Head Diameter, 2.5 mm (73-0711)
• Left Arial Cannula, DD 4.0 mm (73-0712)
Operating Table (Included in the Core System):
The operating table (73-2488) is specially designed for a stable and easy in situ preparation under most physiological conditions (e.g. stable perfusion pressure). It is attached to the Perspex stand and allows an exploration of the lung without interruption of ventilation and perfusion.
Surgery takes place at a hydrostatically neutral elevation, minimizing physiological hydrostatic pressure pertubations. An included plastic trough collects waste. The table is easily removed for cleaning after the lung is placed in the chamber. The operating table is 300 mm x 195 mm in size with four paw clamps, two thorax retractors, holder for chamber lid with heat exchanger, and ball joint for positioning.
Additional Requirements for a Functional Unit
For a functional unit the core system requires a suitable ventilation system. For only positive ventilation a rat ventilator can be used (e.g., VentElite or the Small Animal Ventilator, Type 683). If positive and sub-atmospheric ventilation isneeded, a special Ventilation Control Module (VCM) is required.
Additional Components
Tracheal Cannulae and Cannulae Sets (required, purchase separately):
• Tracheal Cannula for Rat, OD 2.0 mm, L 14 mm, no Luer Connection (73-3384). Order tubing separately.
• Tracheal Cannula for Rat, OD 2.5 mm, L 17 mm, no Luer Connection (73-3557). Order tubing separately.
• Tracheal Cannula for Guinea Pig, OD 3.0 mm, L 20 mm, no Luer Connection (73-3556). Order tubing separately.
• Tracheal Cannula for Guinea Pig, OD 3.5 mm, L 24 mm (73-3555). Order tubing separately.
Tracheal Cannula Set for Rat (73-4277) includes:
• Tracheal Cannula for Rat, OD 2.0 mm, L 14 mm, no Luer Connection (73-3384)
• Tracheal Cannula for Rat, OD 2.5 mm, L 17 mm, no Luer Connection (73-3557)
• 3-Stop Tygon® E-Lab Tubing (AME 23), 2.54 mm ID, 12/pack, Purple/Orange (73-1838)
• 3-Stop Tygon® E-Lab Tubing (AME 25), 3.17 mm ID, 12/pack, Black/White (73-1839)
Tracheal Cannula Set for Guinea Pig (73-4278) includes:
• Tracheal Cannula for Guinea Pig, OD 3.0 mm, L 20 mm, no Luer Connection (73-3556)
• Tracheal Cannula for Guinea Pig, OD 3.5 mm, L 24 mm (73-3555)
• 3-Stop Tygon® E-Lab Tubing (AME 23), 2.54 mm ID, 12/pack, Purple/ Orange (73-1838)
• 3-Stop Tygon® E-Lab Tubing (AME 25), 3.17 mm ID, 12/pack, Black/White (73-1839)
Note: Cannulae Sets come with the appropriate pump tubing.
If only single cannulae are ordered, also order pump tubing 73-1838 and
73-1839. A complete tube set for the perfusion circuit is available (73-3842)
which includes all necessary tubing and adapters for the IL-2 perfusate circuit.
Standard Options for the Core IPL-2 System (Purchase Separately)
PULMODYN Data Acquisition Software
Online evaluation of a wide range of signals and classical respiration parameters.
Note: Ponemah Data Acquisition & Analysis Software from DSI, a Harvard Bioscience Company is also suitable.
Small Animal Ventilator, Type 683
Allows tidal volumes to be set from 0.5 to 5.0 cc in 0.5 cc increments. A larger cylinder allows tidal volumes of 3.0 to 30 cc in 0.3 cc increments. Respiratory rate can be set from 18 to 150 strokes per minute. Volume and rate settings can be adjusted while the ventilator is running.
Allows tidal volumes to be set from 50 µL up to 5 ml. Touchscreen display and many advanced features.
Special Applications & Options
Addition to Perfuse Mouse Lungs
Components to enable mouse perfused lung experiments on an IPL-2 system.
Direct real-time flow measurement with functional IPL-2 systems.
Venous pressure transducer and amplifier for venous pressure measurement in an isolated perfused lung system.
Enables double occlusion or individual and venous occlusion on IPL-2.
For deoxygenation of blood or buffers containing proteins (e.g. albumin) or erythrocytes.
Delivers CO2 to maintain pH when system is not deoxygenated with N2/CO2 gas mixture.
Permits precise continuous or discontinuous measurement in liquid media or perfusate of these three key parameters: pO2, pH and pCO2.
Measure perfusate temperature in any isolated perfused organ system.
For filtration of recirculated perfusate.
Add-on to deliver aerosols to the isolated lung.
For accurate drug addition using a syringe pump. Additional option for flow controlled drug addition, where flow is measured (or calculated) and a drug must be added in a certain ratio.
Lung Weight Measurement (Edema Balance)
Weight measurement-edema balance option for IPL-2 core system for mouse, rat or guinea pig lungs.